Water Splitting for Chemical Fuel Production
Natural photosynthesis, carried out in green plants and purple photosynthetic bacteria, powers the planet by harnessing solar energy to drive endothermic chemical reactions. These endothermic reactions produce oxygen and sugars, which are used as energy carriers in every facet of modern society. Similarly, by gaining control of chemical systems, scientist can harness solar energy using artificial photosynthesis. Artificial photosynthesis stores 58 kcal/mol of solar energy in chemical bonds by splitting water into oxygen gas and hydrogen fuel.
Molecular Water Splitting Based On Natural Photosynthesis
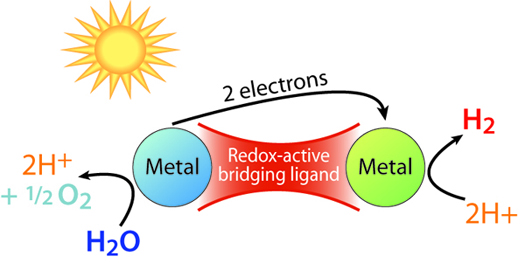
Despite the overwhelming importance of developing such a strategy, little progress has been made towards a workable system for artificial photosynthesis, owing to the slow rates of inter-conversion for the fundamental multi-electron reactions of water splitting. The CfSE is doing fundamental research on developing new multi-metallic systems with redox-active ligands that are desinged to favor multi-electron transformations that need to occur to split water.
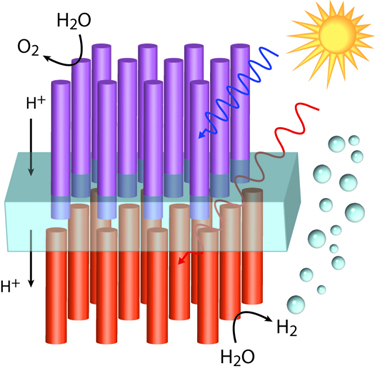
Water-Splitting Devices Using Nanowires
Two-photoelectrode tandem cells are an attractive way to split water because tandem cells provide a larger voltage to drive the chemical reactants while allowing the use of smaller gap semiconductors, resulting in larger photocurrent and higher efficiency. This system requires the absorption of two photons to produce one useful electron-hole pair.
Center Funded Publications
